By Chen Wang (Abbatt Group, University of Toronto) and Doug Collins (Bucknell University, and on Twitter at @EarthMechanic)
The presence of particles suspended in air is associated with negative health effects, including respiratory and cardiovascular issues. While some particles are emitted directly to the air from a source, such as from combustion, formation of new particles through atmospheric chemical reactions is less obvious to the casual citizen. Indoors, we know that extremely small particles (diameters less than 50 nm) are emitted from specific sources like gas stoves, but we don’t know as much about chemical reactions that are specifically prevalent indoors will lead to ultrafine particle formation. Human activities emit a variety of gas phase chemicals: Does indoor chemistry of these gases lead to formation of fine particles? Here, we’ll show two examples of new particle formation arising from two common activities.
Example 1 – Cigarette smoking:
Smoking cigarettes emits a large number of gaseous chemicals and aerosol particles with diameters of 50-400 nm; however, chemical reactions involving cigarette emissions have not been well studied. In particular, cigarette emissions are expected to undergo reactions with oxidants indoors (hydroxyl radical OH and ozone O3) and, potentially, indoor lights. Formation of new aerosol 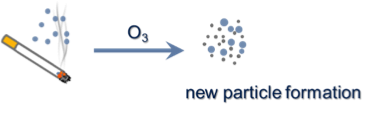 particles normally only happens in environments that lack a large population of pre-existing particles. However, in our laboratory chamber experiments, we found that the reaction of cigarette smoke with O3 always leads to new particle formation, across a wide range of indoor-relevant ozone concentrations. OH also gives rise to new particle formation, if its concentration is high enough. Although shining indoor lights on this mixture did not lead to particle formation, gas phase chemistry can occur, leading to the formation of potentially harmful and chemically reactive compounds, including nitrogen dioxide (NO2) and nitrous acid (HONO). Overall, the indoor atmospheric chemistry of cigarette smoke has the potential to contribute to new particle formation indoors, perhaps exacerbating the health hazard of an already poor environmental scenario.
particles normally only happens in environments that lack a large population of pre-existing particles. However, in our laboratory chamber experiments, we found that the reaction of cigarette smoke with O3 always leads to new particle formation, across a wide range of indoor-relevant ozone concentrations. OH also gives rise to new particle formation, if its concentration is high enough. Although shining indoor lights on this mixture did not lead to particle formation, gas phase chemistry can occur, leading to the formation of potentially harmful and chemically reactive compounds, including nitrogen dioxide (NO2) and nitrous acid (HONO). Overall, the indoor atmospheric chemistry of cigarette smoke has the potential to contribute to new particle formation indoors, perhaps exacerbating the health hazard of an already poor environmental scenario.
Reference on this example:
Wang C, Collins DB, Hems RF, Borduas N, Antiñolo M, Abbatt JPD. Exploring conditions for ultrafine particle formation from oxidation of cigarette smoke in indoor environments. Environmental Science & Technology. 2018;52(8):4623-4631. doi.org/10.1021/acs.est.7b06608
Example 2 – Chlorine cleaning:
People use chlorine bleach for cleaning and disinfection, which emits chlorine-containing gases, such as hypochlorous acid (HOCl) and chlorine(Cl2). 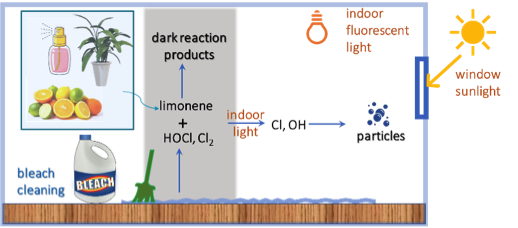 These compounds are very reactive and can either directly react with other indoor volatile organic compounds (VOCs), or can form even more reactive compounds (OH and Cl radicals) when exposed to indoor lights. One group of VOCs commonly found indoors are terpenes, including limonene, arising from use of citrus-scented cleaning products, fragrances, air fresheners, food, and plants. Our research shows that limonene reacts with both HOCl and Cl2 in the dark, but will not lead to new particle formation. However, when indoor lights or window sunlight are available, new particles with a large chlorine component are formed. As recommended, it is best to avoid mixing bleach cleaners with terpene-based products and to keep the room well ventilated during cleaning.
These compounds are very reactive and can either directly react with other indoor volatile organic compounds (VOCs), or can form even more reactive compounds (OH and Cl radicals) when exposed to indoor lights. One group of VOCs commonly found indoors are terpenes, including limonene, arising from use of citrus-scented cleaning products, fragrances, air fresheners, food, and plants. Our research shows that limonene reacts with both HOCl and Cl2 in the dark, but will not lead to new particle formation. However, when indoor lights or window sunlight are available, new particles with a large chlorine component are formed. As recommended, it is best to avoid mixing bleach cleaners with terpene-based products and to keep the room well ventilated during cleaning.
Reference on this example:
Wang C, Collins DB, Abbatt JPD. Indoor Illumination of Terpenes and Bleach Emissions Leads to Particle Formation and Growth. Environmental Science & Technology. 2019;53(20):11792-11800. doi.org/10.1021/acs.est.9b04261