Project Description
In the United States, as well as in most of the developed world, people spend about 90% of their time in indoor environments. Not only are we impacted by the air we breathe and the surfaces we touch, but we also constantly change and influence the indoor environment around us. Although many research efforts have focused on assessing the presence and quantity of chemical air pollutants that affect the indoor air quality, few comprehensive studies have attempted a deeper exploration into how indoor air chemical compounds may interact and transform throughout a normal day of activities.

The HOMEChem experiment (House Observations of Microbial and Environmental Chemistry) took place in the month of June 2018, incorporating measurements from over 20 research groups from 13 universities to identify the most important aspects of the chemistry that controls the indoor environment. The HOMEChem field study kick-started the Chemistry of Indoor Environments community of scientists, while also answering interesting preliminary science questions on the chemistry of indoor environments in a real-world experimental setting. This research effort brings an excellent opportunity for outreach to the broader scientific community and other stakeholders, such as other funding agencies, the local and national media, and the public.
Through this study, we will provide answers to interesting research-based questions using high-end atmospheric chemistry instrumentation, create an opportunity for intercomparing our instruments, and generate a high-quality dataset for the Indoor Chemistry Modeling Consortium (MOCCIE).
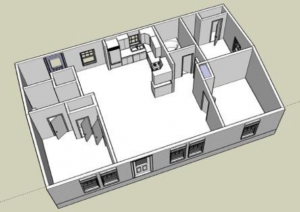
Location
The Manufactured Housing Institute estimates 22 million people live in manufactured homes in the U.S. (about 7% of the population). HOMEChem will take place at the University of Texas at Austin’s test house (the UTest House), which is a 1,200 square-foot manufactured home. This unique facility is one of only a few in the world dedicated solely to indoor environments research.
Science questions
The HOMEChem experiment was centered around three science questions:
- What are the sources of chemical oxidants in the indoor environment, and how are they impacted by changes in light conditions and human activities? Examples of chemical oxidants are hydroxyl radicals (OH), nitrate radicals (NO3-), and ozone (O3), which are highly reactive.
- What are the main sources of organic compounds in the indoor environment? How do human activities lead to changes in the physical and chemical transformation of organic compounds from gaseous state into particulate phase (also known as “secondary organic aerosol”) as well as in other chemical compounds present in minute amounts (also known as trace gas species)?
- What are the sources of indoor reactive nitrogen species, and to what extent is their presence indoors influenced by outdoor pollution?
Experiments
This study is taking place in June 2018. Specifically:
- May 27 – 30: Instrumentation setup
- June 1 – 28: Measurements
- June 29 – 30: Instrumentation take-down

We have chosen a set of experiments that focus on the two most common everyday activities to the air chemistry of indoor environments: cooking and cleaning. These activities are (1) realistic representations of activities performed in indoor environments and (2) likely to drive interesting chemical reactions that can be better understood through this study. The third experimental component of this study relates to occupation: The presence of people in an environment can lead to interesting physical and chemical processes on the surface of clothing and human skin. Additionally, the use of common personal care products and their effect on the chemistry of indoor environments will also be investigated.
We will perform two main categories of experiments:
- “Sequential experiments,” which consist of performing individual and independent activities to better understand their emissions and effects on the indoor environment. In this scenario, the house will be opened and we will let air circulate to bring down concentrations in between each experiment.
- “Layered experiments,” which consist of performing cooking and cleaning activities throughout the day (as we would in a normal home situation) to investigate the cumulative effect of their emissions on the air chemistry and to investigate the interactions of emissions (e.g., cooking emissions interacting with cleaning emissions).
Measurements
In the context of these experiments, the HOMEChem team will be employing a suite of instruments designed to detect specific species inside of the UTest House. The targeted species can be organized into three categories: oxidants, volatile organic compounds (VOCs), and particulate matter.
Oxidants are the main driving forces behind the chemical processes that take place in indoor environments. A group of highly reactive compounds including ozone (O3), hydroxyl radicals (OH-), and nitrate radicals (NO3-), oxidants are important because they set the chemistry in motion. By measuring oxidant species during HOMEChem, we can improve our understanding of their distribution and role within indoor environments, and how they drive the chemistry that takes place.

Volatile Organic Compounds (VOCs) are a much broader group of gas-phase species with a wide variety of sources, including the paint on our walls, our cleaning products, our beauty products, our meals, and even our skin. Each of these sources–and many more–can contribute molecules that form a chemical soup around us. VOCs have the potential to react with the oxidants described above to form a variety of different products. The VOCs and their products can range from lighter and more volatile species that are quickly distributed throughout a room, to heavier and stickier species that have the potential to condense onto surfaces like walls, fabric, and humans, or to participate in the formation of particles. Many VOCs and their products have a negative impact on human health. By measuring a large range of Volatile Organic Compounds during HOMEChem, we hope to improve our understanding of VOC sources and fates within the indoor environment.
Particulate matter is made up of solids or liquids suspended in the air around us. Particles can be directly emitted from activities such as frying bacon on the stovetop, or spraying a can of hairspray. They can also be generated during the reaction of VOCs with oxidants as the products become heavier and stickier. This is called secondary organic aerosol (SOA). Because they have different physical properties than VOCs, particles must be measured using different instrumental techniques. Particulate matter also negatively influences human health when inhaled. By measuring particulate matter throughout the HOMEChem campaign, we will improve our understanding of the processes that may be contributing to the formation of particles indoors.
The HOMEChem study will utilize instruments specifically designed to detect oxidants, VOCs across a wide volatility range, and particulate matter. Each measurement will provide one piece of the puzzle that describes the chemistry of indoor environments; together, these clues will begin to reveal a picture that can help us better understand this complex chemistry.
Instrumentation
Some of the instruments used during HOMEChem include:
Particle techniques:
- Multiple aerosol count and size distribution measurements from 1 nm to 10 micrometers in diameter (Vance and colleagues)
- High resolution time-of-flight aerosol mass spectrometer (HR-TOF-AMS, DeCarlo)
- Chemical ionization mass spectrometer (CIMS) with a filter inlet for gases and aerosols (FIGAERO, Hildebrandt Ruiz)
- Extractive electrospray ionization time-of-flight mass spectrometer (EESI-TOF-MS, Jimenez)
- Ultraviolet aerodynamic particle sizer (UVAPS, Goldstein)
Gas-phase techniques:
- Multiple Chemical Ionization Mass Spectrometers (CIMS acetate and iodide,Farmer, Abbatt, Hidebrandt Ruiz)
- Proton-transfer-reaction mass spectrometer (PTR-TOF-MS, Goldstein) for time-resolved concentrations of a wide range of gas-phase volatile organic compounds (VOCs) and oxygenated volatile organic compounds (OVOCs)
Photo: Callie Richmond - Laser-induced fluorescence assay by gas expansion (FAGE, Stephens) for OH, HO2, HO2*
- Semi-volatile thermal desorption aerosol gas chromatography (SVTAG) with online derivatization for measurement of gas- and particle-phase semi-volatile organic compounds (SVOCs)
- Multiple trace gas detectors: CO2, CO, CH4, O3, SO2, NOX (Farmer, DeCarlo, Corsi, Novoselac, Hildebrandt Ruiz)
- Gas chromatography for measurements of VOCs (Farmer)
Surface and other measurements:
- 3D scan and surface microbiome characterization (Knight, Dorrestein)
- Glass surface analysis (Grassian, Corsi)
- Surface swabs for speciated organics and functional groups in particles (Ziemmann, Jimenez)
- Photon fluxes (Kahan)
The Team:
Principal Investigators
- Dr. Marina Vance (co-PI), Assistant Professor, Department of Mechanical Engineering and Environmental Engineering Program, University of Colorado Boulder
- Dr. Delphine Farmer (co-PI), Associate Professor, Department of Chemistry, Colorado State University
The UT Austin team
- Dr. Atila Novoselac, Professor, Department of Civil, Architectural, and Environmental Engineering, University of Texas at Austin
- Dr. Rich Corsi, Professor, Department of Civil, Architectural, and Environmental Engineering, University of Texas at Austin
- Dr. Lea Hildebrandt, Assistant Professor, Department of Chemical Engineering, University of Texas at Austin
Collaborators
- Dr. Peter DeCarlo, Associate Professor, Department of Civil, Architectural, and Environmental Engineering, Drexel University
- Dr. Tara Kahan, Assistant Professor, Department of Chemistry, Syracuse University
- Dr. Philip Stevens, Rudy Professor, School of Public and Environmental Affairs, Indiana University Bloomington
- Dr. Jon Abbatt, Professor, Department of Chemistry, University of Toronto
- Dr. Bill Nazaroff, Daniel Tellep Distinguished Professor, Department of Civil and Environmental Engineering, University of California, Berkeley
- Dr. Allen Goldstein, Professor, Department of Environmental Science, Policy, and Management, Department of Civil and Environmental Engineering, University of California at Berkeley
- Dr. Jose Jimenez, Professor, Department of Chemistry and Biochemistry, University of Colorado Boulder
- Dr. Paul Ziemann, Professor, Department of Chemistry and Biochemistry, University of Colorado Boulder
- Dr. Rob Knight, Professor, Department of Pediatrics and Computer Science & Engineering, and Director, Center for Microbiome Innovation, University of California, San Diego
- Dr. Pieter Dorrestein, Professor, Departments of Pharmacology and Pediatrics, University of California, San Diego
- Dr. Vicki Grassian, Distinguished Professor, Departments of Chemistry & Biochemistry, Nanoengineering and Scripps Institution of Oceanography, University of California, San Diego
- Dr. Krystal Pollitt, Assistant Professor, Environmental Health Sciences, University of Massachusetts
- Dr. John Spengler, Akira Yamaguchi Professor of Environmental Health and Human Habitation, Harvard T.H. Chan School of Public Health
- Dr. Pratim Biswas, Assistant Vice Chancellor & Department Chair, Lucy & Stanley Lopata Professor, Department of Energy, Environmental and Chemical Engineering, Washington University in St. Louis Engineering
- Dr. Michael Hannigan, Associate Professor, Mechanical Engineering, University of Colorado Boulder
- Dr. Rachel O’Brien, Assistant Professor, Chemistry, The College of William & Mary
- Dr. Pramod Kulkarni from the Center for Disease Control (CDC) Division of Applied Research and Technology has generously offered to loan a unique aerosol spark emission spectroscopy (ASES) for measuring concentrations of metals in particulate matter.
Industry Partners
- Airmodus Oy has generously offered to loan their Nano Condensation Nucleus Counter (model A11) for this study. This instrument will be operated by the Vance group.
- Handix Scientific has generously offered to loan multiple units of their Portable Optical Particle Counters (POPS) for this study. These units will be operated by the Farmer group.
- Airboxlab has generously offered multiple units of their Foobot indoor air quality monitor.
HOMEChem Advisory Board
- Dr. Bill Nazaroff, Daniel Tellep Distinguished Professor, Department of Civil and Environmental Engineering, University of California, Berkeley
- Dr. Rich Corsi, Professor, Department of Civil, Architectural, and Environmental Engineering, University of Texas at Austin
- Dr. Jon Abbatt, Professor, Department of Chemistry, University of Toronto
- Dr. Jose Jimenez, Professor, Department of Chemistry and Biochemistry, University of Colorado Boulder
- Dr. Manabu Shiraiwa, Assistant Professor, Department of Chemistry, University of California, Irvine (representative from MOCCIE, the modeling consortium)
Publications
Recently published peer-reviewed papers on the HOMEChem study:
Overview of HOMEChem: House Observations of Microbial and Environmental Chemistry
Aerosol dynamics modeling of sub-500 nm particles during the HOMEChem study
Chemical and physical properties of organic mixtures on indoor surfaces during HOMEChem
Contrasting Chemical Complexity and the Reactive Organic Carbon Budget of Indoor and Outdoor Air
Cooking, Bleach Cleaning, and Air Conditioning Strongly Impact Levels of HONO in a House
Indoor Emissions of Total and Fluorescent Supermicron Particles during HOMEChem
Indoor particulate matter during HOMEChem: Concentrations, size distributions, and exposures
Large Emissions of Low-Volatility Siloxanes during Residential Oven Use
Observations and Contributions of Real-time Indoor Ammonia Concentrations During HOMEChem
Surface emissions modulate indoor SVOC concentrations through volatility-dependent partitioning
Surface reservoirs dominate dynamic gas-surface partitioning of many indoor air constituents
The molecular impact of life in an indoor environment
Spatial and temporal scales of variability for indoor air constituents
Volatile organic compound emissions during HOMEChem
Additional Resources
- Corbett and Grace Lunsford (Home Performance Workshop) youtube playlist with 36 videos about HOMEChem.
- Two videos produced by the UT Austin faculty innovation center can be seen here.
- Click here to see the Professional photos taken for the HOMEChem project (photographer credit: Callie Richmond).